Abstract
Purpose: Glenoid component loosening remains a common mode of failure for total shoulder arthroplasty and has inspired improvements in implant design, instrumentation, and surgical technique. The purpose of this manuscript was to evaluate the incidence of radiolucent lines and glenoid seating on initial postoperative radiographs using a modern pegged-glenoid design, instrumentation, and surgical technique.
Materials and Methods: We performed a retrospective analysis of a consecutive series of 100 pegged-glenoid total shoulder replacements. In cases of excessive glenoid version, the glenoid was asymmetrically reamed to recreate more normal version. Initial postoperative radiographs were evaluated for the presence of radiolucent lines and completeness of glenoid seating. The preoperative glenoid version measured on axial computed tomography (CT) scans was used to compare differences in version among those with complete and incompletely seated glenoids.
Results: The rate of radiolucent lines observed on postoperative radiographs was 0%. Complete glenoid seating (Grade A) was observed in 81 patients (observer 1) and 82 patients (observer 2). Measurements of preoperative CT scans found a higher percentage of abnormal glenoid version for incompletely seated glenoids (47%) than completely seated glenoids (34%) but no significant difference (P = 0.327). The mean preoperative glenoid retroversion for incompletely seated glenoids was 12.1° and 9.1° for completely seated glenoids (P = 0.263).
Conclusions: Modern surgical techniques, surgical instrumentation, and peg glenoid design have facilitated the ability to eliminate radiolucent lines on initial postoperative radiographs with high rates of complete seating of glenoid components. Incomplete seating may be related to incomplete correction of glenoid version.
Level of Evidence: IV, case series.
Keywords: Glenoid component, pegged-glenoid, radiolucent lines, total shoulder arthroplasty
Introduction
Glenoid loosening remains one of the most common modes of failure for total shoulder arthroplasty (TSA). Radiographic loosening of the glenoid has been associated with worsening functional outcomes, [1] worse pain, and inferior abduction strength. [2] In series with >10-year follow-up, radiolucent lines have been reported in nearly 80% of shoulders, with evidence of component loosening in 34%, and a revision rate specifically for glenoid loosening of 7%. [3]
Radiolucent lines have been observed on the initial postoperative radiographs in up to 94% of cases. [4] Significant effort has thus been made at optimizing surgical technique [5],[6],[7],[8] and surgical implant design [9] with the goal of eliminating initial radiolucent lines, lowering the rate of glenoid loosening, and ultimately improving long-term outcomes of TSA.
A critical step of glenoid preparation requires concentric reaming of the glenoid surface to match the back surface of the future glenoid implant. [10],[11],[12] In cases of abnormal glenoid version or eccentric wear, asymmetric reaming of the glenoid is often used to neutralize the glenoid version. [13],[14],[15] However, excessive reaming with violation of the subchondral bone surface can result in glenoid implant subsidence. [16],[17] It has thus been recommended to maximize the glenoid cortical support of the glenoid during preparation. [18] In many cases, this results in acceptance (intentionally or unintentionally) of a region of unsupported prosthesis and radiographs which show incomplete seating of the glenoid component.
The purpose of this manuscript was to evaluate the incidence of radiolucent lines and quality of implant seating found on initial postoperative radiographs using a modern pegged-glenoid design (Turon™ ; DJO Surgical, Austin, TX, USA) and surgical technique. We hypothesized that a low incidence of radiolucent lines and a high rate of complete seating would be observed using this modern implant and surgical technique. In addition, those cases with incomplete seating of the implant would be related to abnormal preoperative glenoid version.
Materials and METHODS
After obtaining Institutional Review Board approval, we performed a retrospective analysis of 100 consecutive TSA using a pegged-glenoid component with a consistent preparation technique performed by a single, high-volume, fellowship-trained surgeon (JL) over a 15-month period (November 2010 to April 2012). Patients were included if a TSA was performed using a pegged-glenoid component. Patients were excluded if a keeled implant was utilized. Preoperative computed tomography (CT) scans were available for 87 patients. All TSA were performed using the identical techniques including biceps tenodesis, complete resection of the labrum and periglenoid releases to facilitate exposure. The surgical technique utilized modern glenoid preparation instrumentation with power reamers, [10] matching jigs for peg holes, thrombin for glenoid peg hole hemostasis, syringe pressurization of cement into pegged holes, additional manual pressurization using a plastic peg pressurizer, [5],[19],[20] cement application to the backside of the component before implantation, [8] and continuous irrigation during cement curing. [21] In cases of excessive glenoid version, the glenoid was asymmetrically reamed in an effort to recreate normal glenoid version. [13],[14],[15] Effort was made to preserve the subchondral bone in all cases. The DJO Turon™ glenoid component (DJO Surgical, Austin, TX, USA), used in this study, is a compression molded four-peg glenoid with a central compression peg and a rough back surface. Glenoid components were selected based on obtaining a consistent mismatch for the humeral head. In the total shoulder system utilized, the humeral head components match the glenoid components. In this study, glenoids were not downsized.
Initial postoperative radiographs (true AP and axillary), taken 10 days after surgery, were evaluated for the presence of radiolucent lines at the bone-cement interface and the seating on the native glenoid based on the classification system established by Lazarus et al. [4] Radiographs were taken in a standardized manner. The true AP view was taken in the standing position where the patient’s scapula was resting on the imaging plate, and the X-ray beam directed perpendicular to the plate. [22] The axillary view was taken with the arm relaxed and positioned in abduction with neutral rotation, such that a clear space was visible between the glenoid and the coracoid anteriorly and the glenoid and the scapular spine posteriorly. [4] All radiographs were de-identified and reviewed in random order. Each set of radiographs was then evaluated by two observers (SB and JL) who were each blinded to patient name and surgical details.
A comparison of preoperative CT scans was then made between glenoids with complete and incomplete seating. CT scans were obtained using a standard technique which created two-dimensional (2D) axial slices oriented perpendicular to the plane of the scapula to properly align the horizontal plane. All preoperative axial CT scans were reviewed by two independent blinded observers (CG and KV) at two separate occasions separated by a 4-week washout period. Glenoid version was measured by selecting the preoperative 2D CT slice just below the coracoid tip. Using the Friedman et al. criteria, the angle between a line drawn from the medial border of the scapula to the center of the glenoid and the line perpendicular to the face of the glenoid was used to calculate glenoid version. [23] The average of these measurements was used to define the glenoid version. Excessive glenoid version was defined as >15° of retroversion and >5° of anteversion based on previous radiographic and geometric findings of normal glenoid version. [21],[24],[25],[26],[27] The average glenoid version and percentage of abnormal glenoids were then compared between those glenoids found to have complete and incomplete seating.
Statistical analysis
Cohen’s κ coefficient was used for calculating inter-rater agreement among blinded observers grading radiolucent lines and glenoid seating. The following guidelines were used as references for agreement: A score of 1.00 indicated perfect agreement; a score above 0.80 indicated excellent agreement; 0.61-0.80 indicated good agreement; 0.41-0.60 indicated moderate agreement; 0.21-0.40 indicated fair agreement; and 0.20 or below indicated poor agreement. [28] Inter-class coefficients were calculated for intra- and inter-rater reliability among blinded observers measuring glenoid version on available preoperative CT scans. Chi-squared analysis was used to assess the difference in an abnormal preoperative glenoid version percentage between seated and unseated glenoids. Finally, independent samples t-tests were used to determine if a difference existed between the amount of preoperative glenoid version between seated and unseated glenoids, and a post hoc power analysis was performed.
Results
The rate of radiolucent lines observed on initial postoperative radiographs was 0%, as all radiographs evaluated by both observers were grade 0. The inter-observer reliability of observing radiolucent lines among blinded observers was found to be perfect (κ = 1.0).
Complete seating of the implant [Grade A, [Figure 1] was observed in 81 radiographs by the first observer and 82 radiographs by the second observer. Incomplete seating involving <25% of the implant [Grade B, [Figure 2] was observed in 17 radiographs by the first observer and 18 radiographs by the second observer. Incomplete seating involving 25-50% of the implant [Grade C, [Figure 3] was only observed by the first observer in two cases. There were no cases of incomplete seating of 50% or greater (Grade D or E). The inter-observer reliability of observing glenoid seating among blinded observers was found to be good (κ = 0.72).
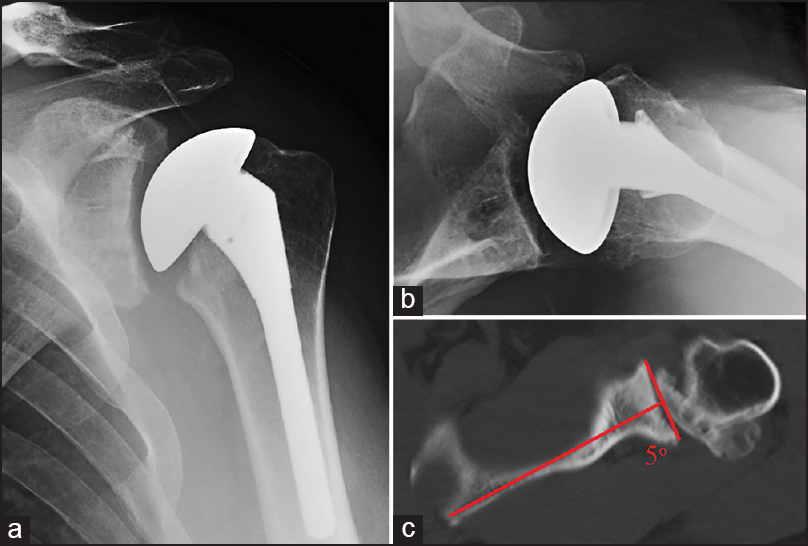 Figure 1: Grade A seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade A glenoid seating following total shoulder arthroplasty
Figure 1: Grade A seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade A glenoid seating following total shoulder arthroplasty
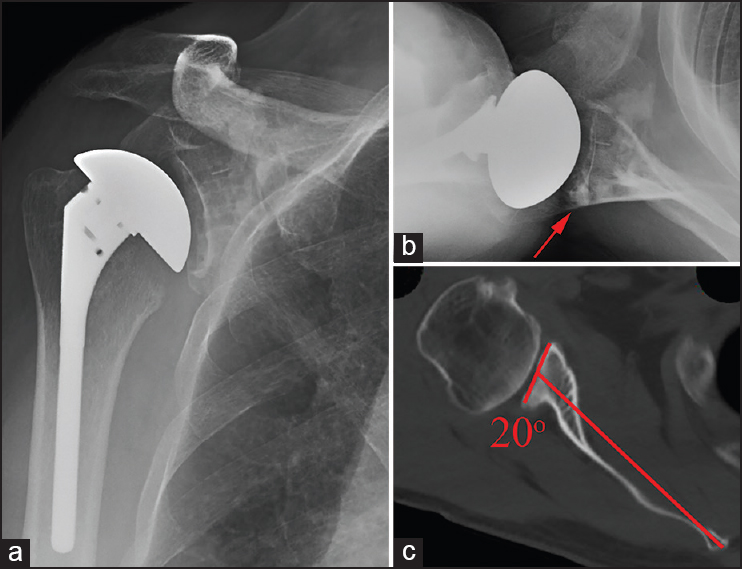 Figure 2: Grade B seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade B glenoid seating following total shoulder arthroplasty. Preoperative retroversion was incompletely corrected, leaving an uncontained area of the posterior glenoid which was filled with cement (red arrow). Incomplete contact was noted in just over 25% of the glenoid seen on the axillary radiograph
Figure 2: Grade B seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade B glenoid seating following total shoulder arthroplasty. Preoperative retroversion was incompletely corrected, leaving an uncontained area of the posterior glenoid which was filled with cement (red arrow). Incomplete contact was noted in just over 25% of the glenoid seen on the axillary radiograph
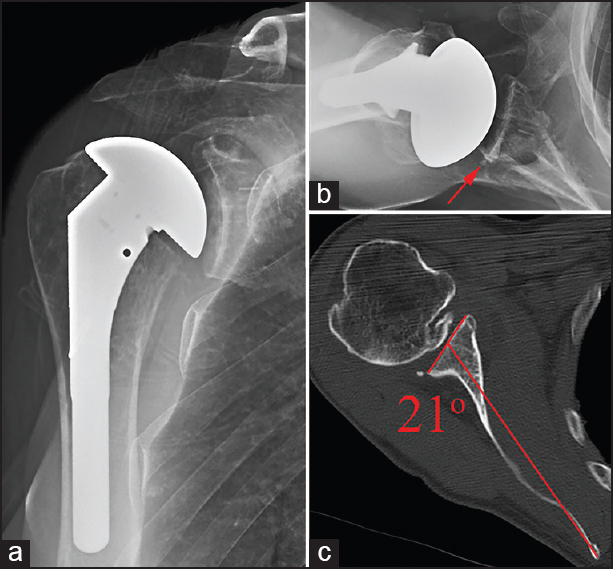 Figure 3: Grade C seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade C glenoid seating following total shoulder arthroplasty. Preoperative retroversion was incompletely corrected, leaving an uncontained area of the posterior glenoid which was filled with a wedge of cement (red arrow) encompassing just under 50% of the glenoid surface seen only on the axillary radiograph
Figure 3: Grade C seating – Immediate postoperative anteroposterior radiograph (a), axillary radiograph (b), and preoperative axial computed tomography scan with measured retroversion (c) illustrating an example of Grade C glenoid seating following total shoulder arthroplasty. Preoperative retroversion was incompletely corrected, leaving an uncontained area of the posterior glenoid which was filled with a wedge of cement (red arrow) encompassing just under 50% of the glenoid surface seen only on the axillary radiograph
Evaluation of the preoperative axial CT scans among two independent blinded observers found excellent inter and intra-observer reliability of glenoid version measurements. The intra-observer reliability for observer one (ICC = 0.982) and observer two (ICC =.950) were excellent. The inter-observer reliability between observers was also excellent (ICC = 0.982).
Preoperative CT scan analysis found the mean glenoid version for patients with completely seated glenoids to be 9.1° of retroversion. For patients with incompletely seated glenoids, the mean retroversion was 12.1°. No statistical difference was found between the mean preoperative glenoid version among patients with complete and incomplete glenoid seating (P = 0.263).
Patients with incompletely seated glenoids were found to have abnormal preoperative glenoid version in 47.1% of cases (8 of 17). Those patients with completely seated glenoids were found to have abnormal preoperative glenoid version in 34.3% of cases (24 of 70). There was no statistically significant difference between the rate of abnormal preoperative glenoid version between these two groups (P = 0.327). The post hoc power analysis performed using these data found that the statistical comparison of version between seated and unseated glenoids yielded a power of 0.244.
Discussion
Our study demonstrated that using modern surgical techniques, instrumentation, and a modern peg glenoid design, the initial rate of glenoid radiolucent lines can be negligible. Much has been learned from previous works that have examined the role of different surgical techniques on lowering radiolucent lines. The technique used in this series was developed from a culmination of evidence on minimizing radiolucent lines. This included the use of power reamers to create a surface matched to the glenoid component, [10] cement pressurization with a syringe, as well as a pressurization instrument, [5],[19],[20] placement of cement onto the backside of the glenoid, [8] and irrigation of the glenoid during cement curing to help negate thermal bone necrosis. [21] Each of these factors likely contributed to limiting postoperative radiolucent lines. In addition, the implant selected was a modern peg glenoid which has been shown to have better initial rates of radiolucent lines than keeled components. [9] While it is impossible to predict the effect that limiting initial radiolucent lines will have on long-term outcomes, it is certainly an indication of improved glenoid component implantation.
While a similar low rate of initial radiolucent lines was reported using a peg glenoid design, [9] this is the first study to additionally look at the influence glenoid version correction has on implant seating. Modern glenoid preparation has evolved substantially since original techniques that utilized hand burr preparation or simple removal of cartilage with a curette. [10] The technique utilized in this series emphasized a goal of concentric reaming of the glenoid bone using power reamers which matched the backside curvature of the glenoid component while maintaining the subchondral bone support. [10],[11],[12],[18]
Irregularities of the glenoid surface and corrections of glenoid version with asymmetric reaming [13],[14],[15] can be accomplished using the modern instrumentation and surgical technique used in this series. However, it is critical to avoid violation of the subchondral plate during reaming, as this lack of the cortical support has been shown to result in implant subsidence. [16],[17] While the ultimate goal is to completely neutralize the glenoid version, [13],[14],[15] this is not always possible due to lack of glenoid bone stock [29] and risk of violating a substantial portion of the subchondral plate. Thus, there are cases where a region of unsupported prosthesis remains, as noted on postoperative radiographs which show incomplete seating of the glenoid component. In this series, complete seating was noted in >80% of cases. In those cases where incomplete seating was observed, there was a higher percentage of excessive preoperative glenoid version (47.1% vs. 34.3%). This did not reach statistical significance; possibly related to the limited number of patients with unseated glenoids. Cases of incomplete seating may represent surgical scenarios where complete concentric glenoid preparation was not possible due to available bone stock and risk of violating the subchondral plate of the glenoid.
This study was limited by a small number of patients with incomplete glenoid seating. While it has been demonstrated that excessive preoperative glenoid retroversion may influence glenoid component implantation and ultimate outcomes, [26],[30],[31],[32] this study did not show preoperative glenoid version statistically influenced the ability to obtain complete glenoid component seating. In addition, patient glenoid size was not taken into account in this study. Patients with excessive glenoid retroversion and smaller sized glenoids may have higher rates of incomplete seating, as asymmetric reaming with preservation of the subchondral bone may be more limited in these patients. Since the size was not controlled in this study, the influence of glenoid size on glenoid component seating cannot be described.
Our study was limited to immediate postoperative radiograph analysis, and was not designed to evaluate the progression of radiolucent lines which has been reported to increase with time. [30] In addition, the study design did not utilize clinical outcomes as an endpoint and thus cannot make any associations with the ultimate clinical improvements. As a single surgeon experience, the results of this study may not be universally reproducible. Finally, as the post hoc power analysis suggests, with a larger cohort of patients with incomplete seating of the glenoid component, it may be possible to see significant differences between preoperative glenoid version and glenoid component seating. Nonetheless, the strengths of this study are the high inter-observer reliability for estimation of preoperative glenoid version, detection of postoperative radiolucent lines, and determination of glenoid component seating using standard radiographic images among blinded observers. The long-term clinical results of this series will be followed closely to evaluate the functional and radiographic outcomes using this technique.
Conclusions
Modern surgical techniques, instrumentation, and peg glenoid design have facilitated the ability to limit the incidence radiolucent lines on initial postoperative radiographs and achieve high rates of complete seating of glenoid components. Incomplete seating may be related to preoperative glenoid anatomy and incomplete correction of glenoid version.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
