Department of Trauma and Orthopaedics, University Hospitals of Morecambe Bay NHS Foundation Trust, Royal Lancaster Infirmary, Lancaster, United Kingdom
Correspondence Address
Abstract
Mycobacterium malmoense is an acid-fast non-tuberculous organism that most commonly causes pulmonary infection. Extrapulmonary infection has also been reported. With an increased emphasis being placed on the clinical importance of this organism, especially within Europe, we report the first case of septic arthritis of the shoulder caused by this organism. We also highlight the importance of considering atypical mycobacterium infection in the differential diagnosis of shoulder infection and issues surrounding the management of this entity.
Introduction
Mycobacterium malmoense is an acid-fast environmental non-tuberculous mycobacterium (NTM) that rarely causes disease in humans but is becoming increasingly clinically significant, especially within Europe. [1],[2] It was first described in 1977 by Schroder and Juhlin, [3] following isolation in respiratory tract culture samples. The most common site of infection caused by this organism is the pulmonary system. [4] However, extrapulmonary infection within lymph nodes, joints, and tendon sheaths has been reported. [1],[2],[4],[5],[6],[7],[8],[9],[10] NTM may develop within the joint as a result of injection or aspiration. [9] M. malmoense infections are seen in both immunocompetent and immunocompromised patients. The latter can be as a result of long-term corticosteroid therapy. [4],[5],[7],[8],[9],[10] Diagnosis has improved over the years and this may account for an increase in incidence of this pathogen. [1],[5] Ziehl-Neelsen (ZN) staining has been used to detect NTM. However, a negative ZN stain does not argue against a NTM infection and extended microbiological cultures are essential for diagnosis. [4],[6] Treatment of extrapulmonary M. malmoense varies, with some authors using stand-alone antibiotic therapy [10] whereas others using surgical intervention, such as debridement, as well as antibiotic therapy. [4],[6]
We report the first case of M. malmoense septic arthritis of the shoulder and conclude that M. malmoense infection should be considered in the differential diagnosis of patients rendered immunocompromised, especially following shoulder procedures.
Case Report
In May 2009, a 60 year-old female on regular prednisolone and methotrexate for focal myositis and vasculitis presented to her rheumatologist with a 1-year history of right shoulder pain and swelling over the right acromioclavicular joint (ACJ). She underwent a subacromial aspiration in clinic, which helped relieve her symptoms. The aspiration showed no growth. Four weeks later, the swelling increased in size and she was again aspirated in clinic. Following this, it began discharging and swabs were taken. The swab results showed no growth. Following two courses of flucloxacillin, her symptoms resolved. In June 2010, she was admitted after developing a discharging abscess anterior to the right ACJ. Investigations revealed that both her C-reactive protein (CRP) and estimated sedimentation rate (ESR) were raised at 82.7 mg/L and 89 mm/h, respectively. A radiograph was taken [Figure 1]. Wound swabs and shoulder joint aspirations were taken. A microbiology opinion was sought and she was commenced on oral rifampicin 300 (mg) twice daily (bd) and intravenous flucloxacillin 1 g four times a day (qds). She failed to improve and was commenced on oral sodium fusidate 500 mg three times a day (tds) and oral ciprofloxacin 500 mg bd. Following a rheumatologic consultation, methotrexate was stopped and prednisolone was continued. A magnetic resonance imaging (MRI) scan of the shoulder was performed [Figure 2]. Following referral to a shoulder surgeon, she underwent an arthroscopic washout of the shoulder joint, open lateral clavicle excision, and closure of wound. In view of no frank pus being observed, no drains were used. Extended cultures were requested. Multiple tissue biopsies were taken, and joint fluid was sent to the laboratory. She was commenced on oral ciprofloxacin 500 mg bd and rifampicin 600 mg bd. The latter had to be stopped due to gastrointestinal disturbance. Initial microbiology and histopathology results were negative for gram stain, ZN stain, Wade-fite stain, and periodic acid-Schiff (PAS) stain. There were foci of granulomatous inflammation. Following surgery, there was a clinical and biochemical improvement. In view of the negative results from microbiology and histology, antibiotics were stopped. Eight weeks later, M. malmoense was isolated which was sensitive to ethambutol, rifampicin, and clarithromycin. There was resistance to isoniazid and ciprofloxacin. Ethambutol 1 g once a day (od) and oral clarithromycin 500 mg bd were commenced. Twelve months following surgical treatment, the patient had clinically, functionally, and biochemically improved. In July 2011, the patient developed bilateral central scotoma secondary to ethambutol toxicity. Ethambutol was stopped with continuation of clarithromycin. She continues to be followed up with normal shoulder function and sinus resolution. However, her ocular symptoms still persist.
 Figure 1: AP radiograph of the shoulder showing a complete loss in the articular surface of the lateral end of the clavicle and resorption of the bone, with prominent swelling above the ACJ
Figure 1: AP radiograph of the shoulder showing a complete loss in the articular surface of the lateral end of the clavicle and resorption of the bone, with prominent swelling above the ACJ
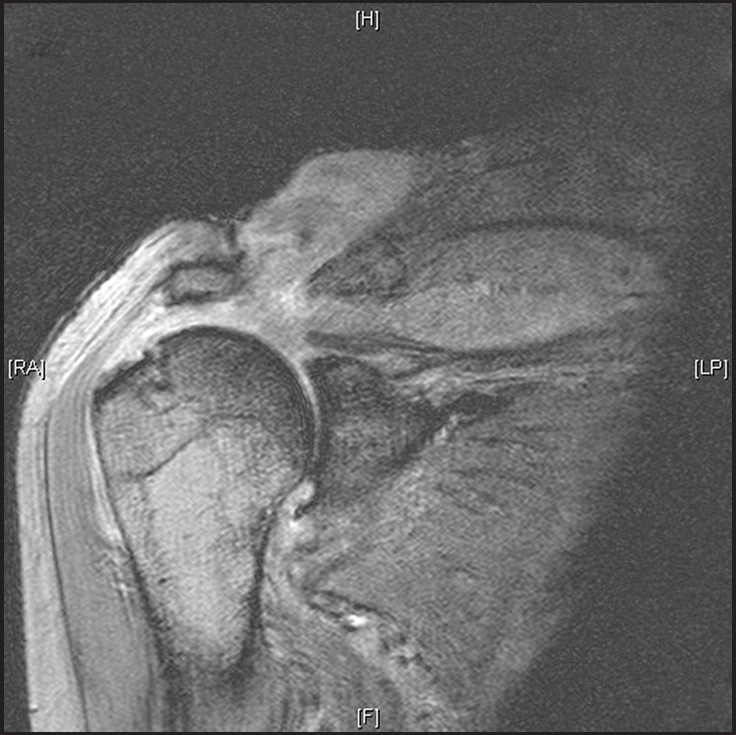
Figure 2: MRI scan showing a large glenohumeral joint effusion with communication with the ACJ via a large rotator cuff defect
Discussion
M. malmoense is a relatively uncommon slow-growing (NTM), first described in 1977 by Schroder and Juhlin. [3] It is an opportunistic pathogen with an increasingly recognized clinical importance worldwide, especially in Europe, and is second only to the Mycobacterium avium complex as a cause of atypical mycobacterial infection. [1],[2] M. malmoense most commonly causes pulmonary disease. In addition to this, extrapulmonary cases have been reported in both immunocompromised and immunocompetent patients. [1],[2],[4],[5],[6],[7],[8],[9],[10] However, no case of isolated shoulder joint involvement with M. malmoense has been reported. Granulomatous infection caused by NTM may develop in tendon sheaths and joints after direct inoculation of the organisms through puncture wounds or injections including intraarticular or bursal steroids. [9] Disseminated NTM disease is very rare with any form of immunosuppression other than advanced HIV disease. However, dissemination of NTM in adult patients without AIDS has been reported in immunosuppressed patients with renal or cardiac transplantation, chronic corticosteroid use, and leukaemia. [1],[2],[9] Early diagnosis of NTM can be difficult and may require recurrent aspiration and referral to a highly specialized laboratory for diagnosis. [7] M. malmoense is an acid-fast organism and ZN staining is positive in the majority of cases. [1],[2] However, negative results have been reported. [4],[6] The latter was a consistent finding with our case. Issues around specific diagnosis can lead to a delay in correct treatment regimes and can lead to pain and disability. It is therefore recommended that repeated cultures are obtained. [7] Furthermore, with the advent of molecular tools, especially 16S rDNA gene sequence analysis, the identification process has sped up and improved. [1] However, it is still recommended that mycobacterial culture for extended periods is the gold standard for diagnosis. [6] M. malmoense infection can be difficult to treat. First-line treatment for the disease is 18 months treatment with rifampicin and ethambutol. [1],[9] However, this is in relation to pulmonary M. malmoense infection and not joint involvement. Treatment of extrapulmonary M. malmoense infection has been described. [4],[5],[6],[7],[8],[9],[10] Different treatment regimes have been reported including; oral antibiotic treatment alone and surgical intervention (debridement and washout) coupled with antibiotic therapy. Both report satisfactory results. However, despite prolonged treatment with antibiotics within the immunocompromised, symptoms may persist and may require surgical exploration and debridement. [7] Either way, there is no randomized control trial found in the current literature, recommending a particular management option for the treatment of M. malmoense infection of the joint. It is also important to note that both ethambutol and rifampicin have significant side effect profiles, including ocular neuritis and gastrointestinal disturbance, respectively. [9] These were both seen in our case, which may lead to suboptimal antimicrobial therapy. In conclusion, our follow-up continues due to incomplete resolution of symptoms and side effects, which is a limitation of the report. However, we believe our case highlights several important issues. To our knowledge, this is the first reported case of M. malmoense infection within the shoulder, and we believe that infection with M. malmoense should be considered as a differential diagnosis in patients rendered immunocompromised, especially following previous injections to, or aspiration of the shoulder. Moreover, we recommend that aspiration of the joint in the immunocompromised be performed in a clean environment other than clinic. Additionally, in view of the difficulties encountered with diagnosis, an early referral to a specialist competent to perform surgical debridement, biopsies, and washout should be considered to ensure adequate tissue samples are obtained. Finally, this case highlights the side effect profile of long-term antibiotic therapy leading to significant morbidity. We suggest regular multidisciplinary follow-up to ensure this is recognized and managed in what is an increasingly common entity.
